

New findings on the lithium-oxygen battery: Overpotential and its causes
source link: https://techxplore.com/news/2023-06-lithium-oxygen-battery-overpotential.html
Go to the source link to view the article. You can view the picture content, updated content and better typesetting reading experience. If the link is broken, please click the button below to view the snapshot at that time.

New findings on the lithium-oxygen battery: Overpotential and its causes
by Christian Wißler, Bayreuth University
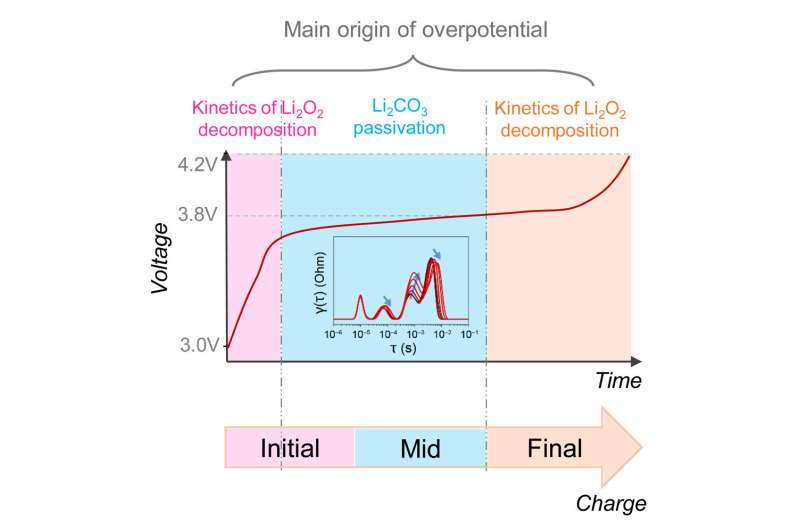
Lithium-oxygen batteries, often hailed as the future of rechargeable energy storage, presently face limitations that prevent their widespread adoption. One of these significant constraints is the occurrence of large overpotentials experienced during the charging process.
This means that the voltage needed for charging increases substantially implying low efficiency. In a new study published in the journal Chem, Prof. Dr. Francesco Ciucci of the University of Bayreuth and research partners in China have for the first time been able to identify and explain the causes of these overpotentials.
The research results can help accelerate the development of more effective and efficient lithium-oxygen batteries and other rechargeable batteries. This is because the factors that are causally involved in overpotential can now be clearly distinguished from one another. In particular, these include the long-term kinetics of lithium peroxide (Li2O2) oxidation and surface passivation by lithium carbonate (Li2CO3). Electrochemical impedance spectroscopy (EIS) is able to monitor these processes separately during battery charging.
"In our study, we integrated electrochemical impedance spectroscopy, using the distribution of capacitive times and the distribution of relaxation times, alongside in situ differential electrochemical mass spectrometry. This enabled a temporally resolved examination of the charging mechanism within an established model catalyst deployed as a representative LiO2 battery electrode," says Prof. Dr. Francesco Ciucci, who holds a professorship in electrode design for electrochemical energy storage at the University of Bayreuth.
He is a member of the Bavarian Center for Battery Technology (BayBatt). The research work published in Chem demonstrates the efficiency of this research approach. At the same time, however, the authors emphasize that further experimental studies are needed to further establish the mechanism.
More information: Juan Chen et al, Charging processes in lithium-oxygen batteries unraveled through the lens of the distribution of relaxation times, Chem (2023). DOI: 10.1016/j.chempr.2023.04.022
Recommend
About Joyk
Aggregate valuable and interesting links.
Joyk means Joy of geeK