

Trichloroethylene: An Invisible Cause of Parkinson’s Disease? - IOS Press
source link: https://content.iospress.com/articles/journal-of-parkinsons-disease/jpd225047
Go to the source link to view the article. You can view the picture content, updated content and better typesetting reading experience. If the link is broken, please click the button below to view the snapshot at that time.

Trichloroethylene: An Invisible Cause of Parkinson’s Disease?
Abstract
The etiologies of Parkinson’s disease (PD) remain unclear. Some, such as certain genetic mutations and head trauma, are widely known or easily identified. However, these causes or risk factors do not account for the majority of cases. Other, less visible factors must be at play. Among these is a widely used industrial solvent and common environmental contaminant little recognized for its likely role in PD: trichloroethylene (TCE). TCE is a simple, six-atom molecule that can decaffeinate coffee, degrease metal parts, and dry clean clothes. The colorless chemical was first linked to parkinsonism in 1969. Since then, four case studies involving eight individuals have linked occupational exposure to TCE to PD. In addition, a small epidemiological study found that occupational or hobby exposure to the solvent was associated with a 500% increased risk of developing PD. In multiple animal studies, the chemical reproduces the pathological features of PD.
Exposure is not confined to those who work with the chemical. TCE pollutes outdoor air, taints groundwater, and contaminates indoor air. The molecule, like radon, evaporates from underlying soil and groundwater and enters homes, workplaces, or schools, often undetected. Despite widespread contamination and increasing industrial, commercial, and military use, clinical investigations of TCE and PD have been limited. Here, through a literature review and seven illustrative cases, we postulate that this ubiquitous chemical is contributing to the global rise of PD and that TCE is one of its invisible and highly preventable causes. Further research is now necessary to examine this hypothesis.
INTRODUCTION
The number of people with Parkinson’s disease (PD) has more than doubled in the past 30 years [1] and, absent change, will double again by 2040 [2]. Numerous genetic causes or risk factors for the disease have been identified, but the vast majority of individuals with PD do not carry any of these mutations [3, 4]. Several environmental toxicants, especially certain pesticides [5], have also been linked to PD, and head trauma is also associated with an increased risk [6]. However, these are insufficient to explain the widespread prevalence of PD. Given the disease’s growing rates— more than can be explained by aging alone [1]— other less visible causes must be contributing to its rise. One of these may be trichloroethylene (TCE), a ubiquitous chemical that has contaminated countless sites and poses health risks to those who are (often unknowingly) exposed via their work or their environment.
The evidence linking TCE to PD to date is based on a handful of case studies [7–12], a small epidemiological study linking exposure to a 500% increased risk of PD [11], and numerous animal studies demonstrating that the chemical leads to the pathological hallmarks of PD [8, 9, 13–17]. Here we introduce the chemical, describe its association to PD and other diseases, detail its widespread use and routes of contamination, and provide circumstantial evidence for its broader role in PD through illustrative cases depicting individuals with the disease who were likely exposed to TCE through their environment or occupation. We conclude with a call for greater research on its effects on PD, protection from and remediation of contaminated sites, and banning of this century-old chemical that has caused immeasurable harm to the public’s health.
WHAT IS TRICHLOROETHYLENE?
TCE is a simple six-atom (two carbons, one hydrogen, and three chlorines) solvent that is clear, colorless, volatile, nonflammable, and environmentally persistent (Fig. 1a) [18]. It was first synthesized in the lab in 1864 (Fig. 1b), and commercial production began in the 1920s [19]. Because of its unique properties, TCE has had countless industrial, commercial, military, and medical applications. Among these are producing other chlorinated compounds (e.g., refrigerants), cleaning electronics, and degreasing engine parts for civilian and military purposes [18]. As it readily evaporates and does not shrink fabrics, TCE was used to dry clean clothes beginning in the 1930s. A closely related chemical called perchloroethylene (PCE), which has one additional chlorine atom in place of the hydrogen atom, largely supplanted TCE in dry cleaning in the 1950s. In anaerobic conditions, PCE often transforms into TCE, and their toxicity may be similar [20].
Fig. 1b
The history of trichloroethylene (TCE) [15, 85]. EPA, Environmental Protection Agency; FDA, Food and Drug Administration.
![The history of trichloroethylene (TCE) [15, 85]. EPA, Environmental Protection Agency; FDA, Food and Drug Administration.](https://content.iospress.com/media/jpd/2023/13-2/jpd-13-2-jpd225047/jpd-13-jpd225047-g001b.jpg)
TCE is found in numerous consumer products (Table 1), including typewriter correction fluid, paint removers, and carpet cleaners [18]. Until the 1970s, it was used to decaffeinate coffee [18]. The volatile TCE was also an inhaled anesthetic until the U.S. Food and Drug Administration banned it in 1977 [19].
Table 1
Historical usage of trichloroethylene [19, 72, 73, 85–88]
| Commercial &Consumer Products |
| Adhesives* |
| Aerosol cleaning products* |
| Carpet cleaner* |
| Cleaners and solvent degreasers* |
| Cleaning wipes* |
| Cosmetic glues |
| Decaffeinated coffee |
| Film cleaners |
| Glue |
| Gun cleaner |
| Fumigant |
| Hoof polishes |
| Inks |
| Lubricants |
| Mold release |
| Paint and paint removers* |
| Pepper spray |
| Pesticides |
| Refrigerant* |
| Sealants |
| Stain removers* |
| Tap and die fluid |
| Toner aid |
| Tool cleaners |
| Typewriter correction fluids* |
| Wood finishes* |
| Industry Usage |
| Automotive care |
| Dry cleaning* |
| Degreasing* |
| Furniture care |
| Manufacturing |
| Computer and electronics |
| Disinfectants |
| Dyes |
| Fat and oil extraction |
| Flavor extracts (spices, hops) |
| Jewelry |
| Machinery* |
| Paint and coating* |
| Paper |
| Perfumes |
| Plastics |
| Refrigerant* |
| Soaps |
| Medicine |
| Anesthesia (medical, dental, veterinary) |
| Surgical disinfectant |
| Treatment (migraines, trigeminal neuralgia) |
| Pharmaceutical manufacturing |
*Common current uses.
TCE AND PARKINSON’S DISEASE
Studies (Table 2) linking TCE exposure to PD and parkinsonism date back to at least 1969 when Huber reported parkinsonism in a 59-year-old man who worked with TCE for over 30 years [7]. Thirty years later, Guehl and colleagues documented PD in a 37-year-old woman who was exposed to the chemical while cleaning houses and again while working in the plastics industry [8]. In 2008, Gash and colleagues reported that among 30 factory workers, three developed PD after using TCE for many years to degrease and clean metal parts [9]. These three workers were stationed closest to an open TCE vat, and 14 of 27 workers who were further from the source “displayed many features of parkinsonism, including significant motor slowing” [9].
Table 2
Clinical studies linking trichloroethylene and parkinsonism or Parkinson’s disease [7–12]
| Authors | Year | Ref | Study Design | N | Findings |
| Huber | 1969 | [7] | Case study | 1 | A 59-year-old that worked with TCE for 33 years developed parkinsonism. The patient’s brain section showed depigmentation and severe degenerative changes in the substantia nigra. |
| Guehl et al. | 1999 | [8] | Case study | 1 | A former house cleaner was exposed to TCE for several months, beginning at age 27, in poorly ventilated rooms. She then worked for six years in the plastics industry in a very small, unventilated office exposed to TCE and other volatile compounds. Three years later, she was diagnosed with PD at the age of 37. |
| Kochen et al. | 2003 | [12] | Case series | 3 | Three workers chronically exposed to TCE developed PD in the post-exposure period. |
| Gash et al. | 2008 | [9] | Case series | 3 | Three industrial plant workers (ages 49, 76, 56) developed PD after years of dermal and respiratory exposure (exposure duration 25 years, 25 years, 29 years) from cleaning metal gauges in a large, open vat of TCE. An additional 14 coworkers in this cluster that experienced chronic respiratory exposure to TCE exhibited parkinsonism features. |
| Goldman et al. | 2012 | [11] | Case-control study in twin pairs discordant for PD | Both occupational and hobby exposure to PCE and TCE among a cohort of twins was studied. TCE was associated with a significantly increased risk of PD (OR 6.1, 95% CI 1.2 – 33; p = 0.034) along with PCE exposure suggestive of an increased risk (OR 10.5, 95% CI 0.97 – 133; p = 0.053). | |
| Reis et al. | 2016 | [10] | Case study | 1 | A former car repairman who worked with products containing TCE for over 40 years was diagnosed with PD at the age of 57. |
PD, Parkinson’s disease; TCE, trichloroethylene.
Four years later, researchers found that in twin pairs, the twin with occupational or hobby exposure to TCE had a 500% increased risk of PD (OR 6.1, 95% CI: 1.2–33; p = 0.034) compared to their unexposed twin [11]. Exposure to the closely related solvent PCE also trended toward significance with an odds ratio of 10.5 (95% CI: 0.97–113) [11]. Notably, the researchers found an interval of 10 to 40 years from the time of TCE exposure to PD diagnosis [11].
TCE and PCE likely mediate their toxicity through a common metabolite [21, 22]. Because they are lipophilic [11], both TCE and PCE readily distribute in the brain and body tissues and appear to cause mitochondrial dysfunction at high doses. This may partially explain the link to PD as dopaminergic neurons are sensitive to mitochondrial neurotoxicants such as MPTP/MPP+, paraquat, and rotenone [23]. Indeed, in animal studies (Table 3), TCE treatment caused selective loss of dopaminergic neurons [8, 9, 13, 15, 16]. In addition, PD-related neuropathology, such as neuroinflammation and α-synuclein phosphorylation and accumulation, was observed in the substantia nigra of rats and mice exposed to 200–1000 mg/kg TCE over chronic time periods (6 weeks to 8 months) [13, 15, 17]. While the specific metabolite or mechanism of TCE-induced neurodegeneration remains unclear, pre-clinical studies with high doses (400–1000 mg/kg) showed that mitochondrial complex I activity is dysregulated in the midbrain of rodents exposed to TCE [9, 13–15]. Mitochondrial function was further reduced in the rat striatum when TCE exposure occurred in conjunction with another PD risk factor, traumatic brain injury. The combined neurotoxic insults resulted in 50% reduction in complex I oxygen consumption [14], a more severe effect than each factor alone. This combined effect provides a key example of how TCE exposure may influence PD risk in certain populations, such as individuals who served in the military where head trauma is morecommon [24].
Table 3
Animal studies involving trichloroethylene and Parkinson’s disease [8, 9, 13–17]
| Authors | Year | Ref | Animal | Exposure | Findings |
| Guehl et al. | 1999 | [8] | OF1 mice | 400 mg/kg TCE, 5 days/week for 4 weeks | Dopaminergic neurodegeneration in the substantia nigra |
| Gash et al. | 2008 | [9] | Fisher 344 rats | 1000 mg/kg TCE, 5 days/week for 6 weeks | Mitochondrial complex I activity inhibition in substantia nigra, increased complex I activity in striatum, dopaminergic neurodegeneration in nigrostriatal tract |
| Liu et al. | 2010 | [13] | Fisher 344 rats | 200, 500 or 1000 mg/kg TCE, 5 days/week for 6 weeks | Dose-dependent loss of dopaminergic neurodegeneration in the substantia nigra, motor deficits in 1000 mg/kg TCE-treated rats, mitochondrial complex I inhibition in substantia nigra, elevated oxidative stress markers, activated microglia, and intracellular alpha-synuclein accumulation in dorsal motor nucleus of vagus nerve |
| Sauerbeck et al. | 2012 | [14] | Fisher 344 rats | 1000 mg/kg TCE, daily for 1 or 2 weeks with and without traumatic brain injury | Mitochondrial impairment in striatum, with rates of complex I dependent oxygen consumption decreasing by 75%, after two week exposure to TCE and traumatic brain injury. Analysis of one week of TCE exposure and traumatic brain injury indicated a 50% decrease in mitochondrial function. Motor impairment and dopaminergic neurodegeneration in the substantia nigra |
| Liu et al. | 2018 | [15] | Male C57BL/6 mice and postnatal day 1–3 Sprague-Dawley rat pups | 400 mg/kg/day TCE, 5 days a week for 8 months | Progressive dopaminergic neurodegeneration, decreased dopamine and metabolites, deficits in locomotor activity, mitochondrial complex I inhibition, increased accumulation of phosphorylated a-synuclein, and endogenous formation of toxic metabolite (TaClo) |
| Keane et al. | 2019 | [16] | A30P and wild type mice | 1000 mg/kg TCE, twice weekly for 8 weeks | Dopaminergic neurodegeneration in the substantia nigra |
| De Miranda et al. | 2021 | [17] | Aged, male and female Lewis rats | 200 mg/kg TCE, daily for 3 or 6 weeks | TCE activated LRRK2 kinase activity prior to dopaminergic neurodegeneration in the nigrostriatal tract. Elevated oxidative stress, neuroinflammation, endolysosomal dysfunction and alpha-synuclein accumulation |
TCE, trichloroethylene.
In addition to combined environmental factors, evidence from preclinical studies suggests that genetic risk factors may also play a role in TCE-induced neurodegeneration. For example, in a 2021 study, chronic, systemic exposure to 200 mg/kg TCE elevated the kinase activity of LRRK2 (leucine rich repeat kinase 2) in the striatum and substantia nigra of rats after 3 weeks, prior to the loss of dopaminergic neurons at 6 weeks [17]. Inherited variants of LRRK2 are linked to both familial and sporadic PD, the most common of which is the G2019S mutation, that pathogenically elevate LRRK2 kinase activity resulting in dysregulated vesicular trafficking, endolysosomal dysfunction, and oxidative stress [25]. However, despite cellular dysfunction caused by elevated LRRK2 kinase activity, individuals who inherit the LRRK2 G2019S mutation have only a roughly 50% increased risk for PD [26]. Incomplete penetrance of genetic risk factors suggests that possible gene-environment interactions could explain why only some individuals exposed to TCE develop PD and why those with a PD-related genetic predisposition may display variable risk of developing PD. Many other genetic causes of PD (e.g., Parkin, PINK1) also affect mitochondrial function, and an interaction with TCE is conceivable for carriers of mutations in these genes [27]. However, more data on gene-environment interaction between TCE, LRRK2, and other genetic risk factors associated with PD are needed.
WIDESPREAD USE, WIDESPREAD CONTAMINATION
TCE was “ubiquitous” in the 1970s [28] when annual U.S. production surpassed 600 million pounds per year, or over two pounds per person [29]. About 10 million Americans worked with the chemical or other organic solvents daily; in the U.K. an estimated 8% of workers have (Table 4) [10]. While domestic use has waned, the U.S. is still the top global exporter of TCE, and since 1990, occupational exposure to TCE has increased by 30% worldwide [30]. Exposure is widespread, and a 1994 study in Italy found TCE at relatively high concentrations in the blood and urine of three quarters of a sample of the general population [31].
Table 4
Example occupations where trichloroethylene exposure may occur [85, 86, 90]
| Aircraft maintenance workers |
| Automotive factory workers |
| Communications equipment repairers |
| Computer specialists |
| Corrosive control technicians |
| Distillery workers |
| Dry cleaners |
| Electronic component manufacturers |
| Embalmers |
| Food manufacturers |
| Insecticide manufacturers |
| Jet engine mechanics |
| Leather manufacturers |
| Machinery installation &assembly workers |
| Mechanics |
| Metal treatment workers |
| Missile technicians |
| Nautical equipment workers |
| Oil processors |
| Painters |
| Pesticide manufacturers |
| Pharmaceutical manufacturing factory workers |
| Printers |
| Radar technicians |
| Refrigerant manufacturers |
| Resin workers |
| Rubber cementers |
| Sewerage workers |
| Silk screeners |
| Shoe makers |
| Systems technicians |
| Taxidermists |
| Textile manufacturers |
| Textile and fabric cleaners |
| Tobacco denicotinizers |
| Waste treatment workers |
| Weapons specialists |
| Varnish workers |
Although the European Union and two U.S. states have banned TCE, it is still permitted for vapor degreasing and spot dry cleaning in the U.S. and for authorized industrial uses in the E.U. [32]. Globally, TCE consumption is projected to increase by 3% annually (Fig. 2a) [33], and China, which has the fastest growing rates of PD [1], now accounts for half the global market [34].
Workers can inhale or come in dermal contact with TCE, but millions more encounter the chemical unknowingly through outdoor air, contaminated groundwater, and indoor air pollution. In 1987, nearly 56 million pounds of TCE were released into the air in the U.S. alone (Fig. 3) [35]. TCE can also leak from storage tanks or be dumped into the ground where it contaminates up to one-third of the drinking water in the U.S. [36]. TCE has also polluted the groundwater in at least twenty different countries on five continents (Fig. 2b).
TCE contaminates countless industrial, commercial, and military sites. TCE is found in half of the 1300 most toxic “Superfund” sites that are part of a federal clean-up program, including 15 in California’s Silicon Valley where TCE was used to clean electronics [37]. The U.S. military has stopped using TCE, but numerous sites have been contaminated, including the Marine Corps base Camp Lejeune in North Carolina. For 35 years, the base— which housed a million Marines, their families, and civilians— had levels of TCE and PCE in the drinking water 280 times safety standards [38].
Beginning in 1978, another route of exposure to TCE and other volatile chemicals was recognized: vapor intrusion (Fig. 4). Researchers found that TCE, much like radon, could evaporate from contaminated soil and groundwater and enter homes, schools, and workplaces [39]. Buildings often have lower air pressure than the outdoor environment and can draw toxic fumes through cracks in the foundation, utility lines, duct work, and elevators [40, 41]. This polluted air can travel upwards to apartments and offices located above plumes, which function as underground rivers of pollutant within the groundwater. TCE has been found in the indoor air of homes, in the butter in their refrigerators (TCE and PCE are fat soluble), and in the breast milk of nursing mothers [42].
Fig. 4
Possible modes of exposure to trichloroethylene in the environment.
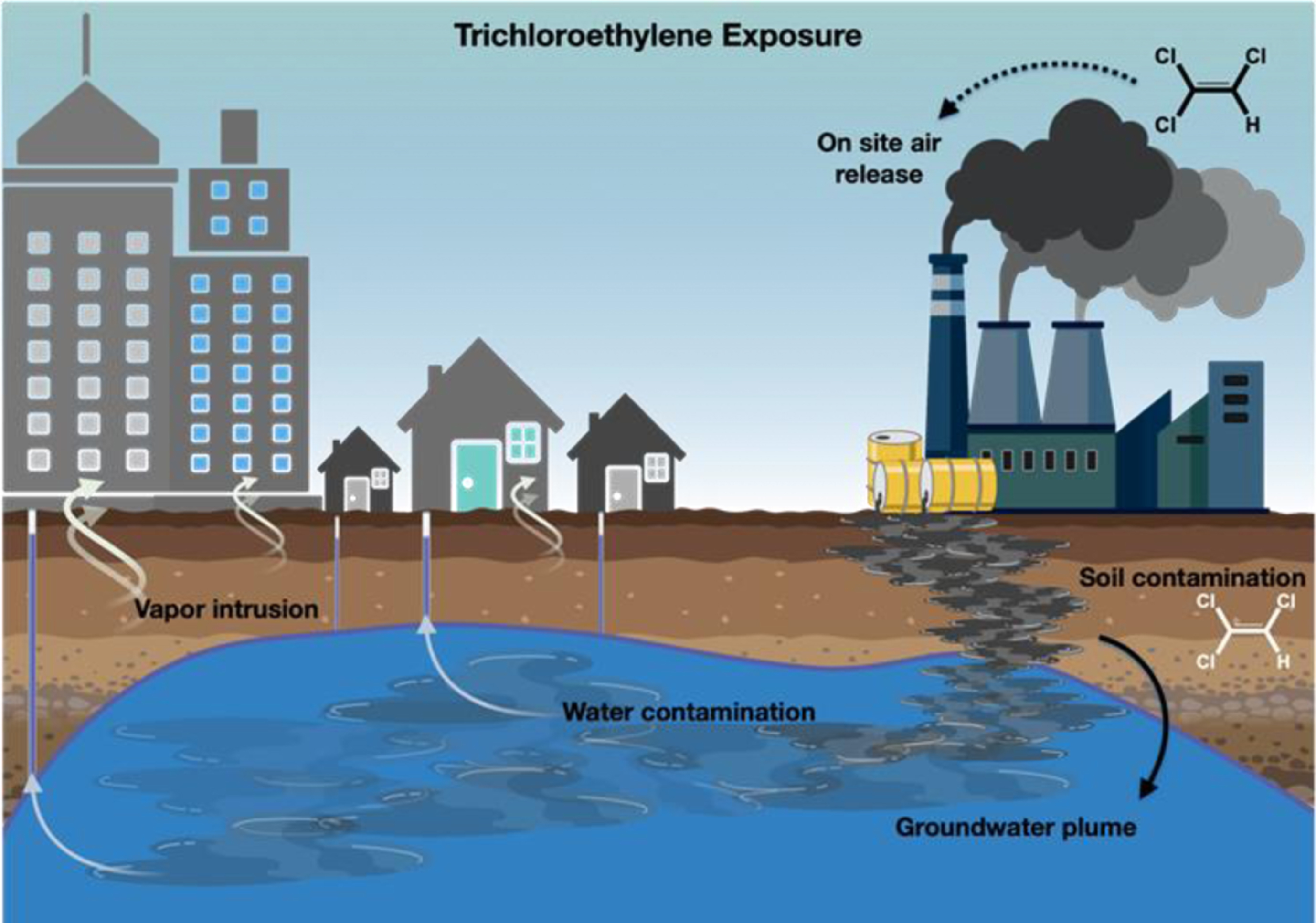
Since contaminated underground plumes can travel over a mile, individuals who live far from a contaminated site are still at risk. One plume on Long Island, New York, which was associated with an aerospace company, is over four miles long and two miles wide and has contaminated the drinking water of thousands [43]. In Shanghai, China, a village, primary schools, and homes sit atop a TCE-contaminated site where a chemical plant operated for over thirty years [44]. In Newport Beach, California, multi-million dollar homes were built above a former aerospace facility known to be contaminated with TCE and PCE [45, 46]. In Monroe County, New York, where many of the authors of this report live, over a dozen dry cleaners have contaminated the ground with TCE.
ILLUSTRATIVE CASES
Below are seven cases where TCE may have contributed to an individual’s PD. The evidence linking possible exposure to TCE in these cases is circumstantial but raises worrisome questions about the link between the chemical and the disease. The first three cases depict likely environmental exposure contributing to PD. The latter four highlight potential risks from occupational exposure. In some cases, identifying information was changed to protect privacy.
Likely environmental exposure to TCE
Case 1
On May 12, 2006, Mr. Brian Grant played two minutes for the Phoenix Suns in a National Basketball Association (NBA) playoff game. He did not score a point, grab a rebound, or have an assist. However, in the last game of his NBA career, the then 34-year-old power forward made history— he had likely just played an entire basketball season with PD.
Mr. Grant first noticed the symptoms of the disease a season earlier while on the Los Angeles Lakers. There the 6’9”, 250-pound player was puzzled to discover he could no longer jump off of his left leg as he once could. Sometimes the leg would give out. The next season, he developed an intermittent tremor in his left hand [47]. Two years later, he was diagnosed with PD.
The roots of his PD may have been in Camp Lejeune [48]. When Mr. Grant was three years old, his father, then a Marine, was stationed at the base around the time that TCE levels in the water peaked [49]. There, Mr. Grant and his family lived in a trailer park on a dirt road. He enjoyed living on the military base, taking a bus to pre-school, and exploring its fighter planes. Mr. Grant also drank, bathed, and swam in the contaminated water, unaware of its toxicity.
Mr. Grant’s PD did not become apparent until about three decades after his family left Camp Lejeune. No one in his large family has had PD. His younger brother who was born on the base suffered disabling allergies that only resolved after they moved away. In March 2020, Mr. Grant’s father died at age 65 from esophageal cancer, which is linked to TCE [50].
Case 2
From 1984 to 1988, a young Navy captain, Amy Lindberg, was also stationed at Camp Lejeune in Jacksonville, North Carolina. On hot, humid days, Captain Lindberg swam, ran, trained, and outworked her peers. She also drank lots of water. What Captain Lindberg did not know is that the water that she drank, bathed, cooked, swam, and played in was contaminated with TCE, PCE, and other toxicants.
Between active duty and the reserves, Captain Lindberg served for 26 years, before she and her husband retired in northern Virginia. In 2017, thirty years after being stationed at Camp Lejeune, the then 57-year-old Captain Lindberg developed anxiety, depression, and trouble thinking (“brain fog”) and was seen by a neuropsychologist. He asked her about her loss of smell, decreased right arm swing, and dragging of her right leg, all of which she had developed about two years earlier. She also had a mild rest tremor in her right hand and long-standing constipation. She soon saw a neurologist who diagnosed her with PD, which was not present in her family.
Now 63, Captain Lindberg remains an avid runner, boxes regularly, and works out frequently, but is disabled by the disease’s non-motor features including urinary urgency, pain, and mood changes. In 2017, the U.S. Department of Veterans Affairs established PD as having a “presumptive service connection” for those who, like Captain Lindberg, served at Camp Lejeune between 1953 and 1987 [51].
Case 3
Dr. Jesh Mittal is a 48-year-old endocrinologist who was raised in an upstate New York community heavily contaminated by TCE. His first home, where he lived until age 14, was located less than a mile from a Superfund site where TCE, PCE, or both had contaminated 60 residential drinking wells [52]. His second home, where he resided until starting college, was also less than a mile from another Superfund site contaminated by TCE and other solvents used in vapor degreasing [53]. However, his potential exposure did not end at home.
The future physician attended high school adjacent to a large computing firm where his father worked. The soil and groundwater at the manufacturing site were contaminated with TCE and PCE. In 1971, seven years before his freshman year, the well at the high school was found to have “slight contamination” with TCE even after a filtration system was installed [54]. A generation later in 2000, groundwater monitoring found high concentrations of PCE at the manufacturing facility [55]. Neither his homes nor his high school were (to our knowledge) ever checked for vapor intrusion despite their proximity to contaminated sites.
In 2010, after a nurse noticed that his handwriting was becoming smaller, the right-handed physician was diagnosed with writer’s cramp. Two years later, he developed constipation, a “twitch” in his right hand, and dystonia in his right arm. He was subsequently diagnosed with PD at age 38. He had no family history of and no genetic marker for PD. Two years earlier, his mother was diagnosed with breast cancer, and three years after his PD diagnosis, his father was diagnosed with prostatecancer.
Likely occupational exposure to TCE
Case 4
Dr. John Smith was an 85-year-old physicist and industry executive with a family history of PD in his father and two paternal aunts, all of whom grew up on a farm. At age six, the future electrical engineer and his family moved to a farm in upstate New York where the young boy would apply rotenone and DDT to green bean plants as part of his chores. As a graduate student, he used TCE to wash electric parts but wore no personal protective equipment.
Upon completing his PhD, he joined National Aeronautics and Space Administration (NASA) where he cleaned electronics and was “swimming” in TCE. His term at NASA was interrupted by basic training in the army at Fort Gordon, Georgia, which served as a testing site for Agent Orange [56]. He then worked for a large computer manufacturing company in East Fishkill, New York, where TCE, PCE, and other chemicals eventually contaminated the soil and groundwater [57].
In approximately 2010, Dr. Smith was diagnosed with PD. His symptoms included anxiety, decreased energy, anhedonia, diminished initiative, depressed mood, and constipation in addition to a rest tremor, slowed movements, a stooped posture, and a soft voice. Some of these symptoms improved with levodopa, but they subsequently worsened. As part of a physical exam in 2019, an internist found a breast lump in Dr. Smith’s chest. The lump was cancerous. The treatment of his breast cancer, which is associated with TCE exposure [58], required surgery and tamoxifen.
Case 5
Mr. Ethan Jones is a 72-year-old retired teacher who was diagnosed with PD in 2017. He also carries a G2019S mutation in LRRK2.
In his early thirties, Mr. Jones worked for three to four years in a small copy and print shop that required multiple chemicals and solvents. He is unsure whether he was exposed to TCE or PCE, but chlorinated solvents were commonly used in the industry in the 1970s and 1980s [59]. About 35 years later, he noticed that he was moving slower than his peers and was subsequently diagnosed with PD at age 68. His symptoms improved with levodopa, which he continues to take.
Neither of his parents had PD, but his paternal grandfather did and his nephew does. In addition to PD, he was diagnosed with monoclonal gammopathy of undetermined significance, a premalignant state associated with multiple myeloma, which is associated with TCE exposure [60].
Case 6
After serving in the military, Mr. Alex Janssen worked in the construction and automotive industry. In these latter jobs, he worked with degreasing chemicals, such as TCE, for approximately seven years. About five years after his exposure ended, he noticed numbness on the right side of his body followed by difficulty walking up the stairs. He later experienced a stressful event that was followed by involuntary shaking in his right arm and leg and a PD diagnosis at age 33.
The number and intensity of PD symptoms increased significantly over the years, and he eventually had deep brain stimulation (DBS), which improved his symptoms and his quality of life. Three years later, he developed fatigue, headache, and a facial droop. Brain imaging at age 53 revealed a stage IV glioblastoma situated next to a DBS wire.
Case 7
In 2020, Georgians took to the polls to elect two U.S. Senators in a closely watched election that would determine political control of the legislative body. The reason for the unusual election? Parkinson’s disease. The late Senator Johnny Isakson, who was diagnosed with PD in 2015, had stepped down due to “health challenges” in 2019, leading to a special election in 2020 [61].
Senator Johnny Isakson, who died in 2021 at age 76, served for fifteen years in the U.S. Senate during which time he was a staunch advocate for veterans and co-chaired the Congressional Caucus on Parkinson’s Disease [62]. In addition to his PD, Senator Isakson had a two-centimeter renal cell carcinoma removed from his kidney in 2019 [61], a tumor associated with TCE exposure [63].
Nearly fifty years before his PD diagnosis, the future Senator served in the Georgia Air National Guard from 1966 to 1972. The military, including the Air Force, used TCE to degrease airplanes during this period [64], and many military bases, including those in Georgia [65], have been contaminated with the chemical [66].
ADDITIONAL TOXICITY OF TCE
As depicted by these cases, the adverse health effects associated with TCE extend far beyond PD. Its toxic effects begin shortly after conception. TCE can cross the placenta, [67] and maternal exposure to TCE is associated with low birth weight [68], congenital heart disease [68], and neural tube defects [69]. At the TCE-contaminated Marine Corps Base Camp Lejeune, at least seven babies had anencephaly, and ten had spina bifida [19, 70]. After birth, TCE-linked diseases proliferate as the solvent is linked to conditions affecting nearly every organ system [71] including cancer.
According to the U.S. Environmental Protection Agency (EPA) and World Health Organization, TCE is carcinogenic to humans by all routes of exposure [72, 73]. A meta-analysis found occupational exposure “was associated with excess incidences of liver cancer, kidney cancer, non-Hodgkin’s lymphoma, prostate cancer, and multiple myeloma, with the strongest evidence for the first three cancers” [74]. This is likely only a partial list. At least 78 men who lived at contaminated Camp Lejeune have been diagnosed with breast cancer [49]. In addition, high rates of brain and other central nervous system tumors have been reported in animal studies [19] and in TCE-contaminated communities [75, 76].
TCE’s adverse health effects have long been known. In 1932, Dr. Carey McCord, a physician working for the Chrysler Corporation, wrote a letter to the Journal of the American Medical Association. He said that activities of TCE “frequently fail to disclose the toxic nature of this chemical and the practical dangers that may attend its use.” He then detailed experiments with rabbits in which repeated skin exposure to the chemical caused death in days. Inhalation of TCE “under conditions of trivial exposure” killed the rabbits in days if not hours. Ninety years ago, he concluded that the solvent could be “the source of disaster for exposed workmen” [77].
CRITIQUE
The cases described demonstrate the potential role that TCE plays in PD. However, they are far from definitive and far from the only ones. The vignettes highlight many of the difficulties in establishing a strong link between the invisible TCE and PD. Among these are the following: 1) many are unaware of their exposure; 2) exposure, if present, was usually unmeasured; 3) previous exposures cannot currently be measured; 4) in many cases, exposure co-occurred with other pollutants; 5) time between exposure and disease is long; 6) underlying genetic risk factors, which are often not assessed, may augment the risk of developing PD following TCE exposure; and 7) diagnosis of PD is often delayed or missed.
In just one of the cases above was the person— a physicist— aware of his exposure to the toxic chemical at the time it occurred, and all were unaware of the health risks associated with the chemical. Those who drank contaminated water or inhaled polluted air outside or inside their workplaces, schools, or homes generally had no idea that they were exposed. Today, sites known to be contaminated with TCE, including many of the most toxic ones in the U.S., have no warning signs, fences, notices, or other public notification of the inherent dangers. As a result, it is challenging to determine whether exposure occurred.
Moreover, if exposure did occur, retrospective exposure assessment of TCE is difficult. Exposure is almost never measured contemporaneously (indeed, we are unaware of any case of PD associated with TCE where it was). Biomarkers of historical TCE exposure do not currently exist. The few studies [9, 20] and case reports [11, 73] available suggest that a dose-response relationship may be present as individuals who work most closely with the chemical have a shorter lag between exposure and disease onset.
Like other environmental toxicants (e.g., smoking, pesticides), exposure to TCE is often combined with other exposures. Many TCE-contaminated sites are polluted with PCE and other toxic hydrocarbons such as benzene and carbon tetrachloride, which itself may be associated with PD.
The effect of each individual compound has often not been assessed, and research into the risk of exposure to mixtures of toxicants is needed [78, 79].
The time between exposure and disease onset may be decades. Individuals, if they were aware of their exposure to the chemical, may have long since forgotten about it. Those who worked with the solvent or who lived near a contaminated site may have changed jobs or moved, making retrospective evaluation of potential clusters challenging.
Finally, while TCE’s effects on cancer are well-documented, its effects on PD are only recently coming to light. Gash’s study of factory workers who developed PD after degreasing metal parts with TCE was published in 2008 [9]. The twin study quantifying the high degree of association between occupational or hobby exposure to TCE and PD is only ten years old [11]. Many individuals who know they were exposed to TCE and subsequently developed PD have no reason to link the two. Today, most clinicians are unaware of TCE’s deleterious health effects even though they have been documented for over ninety years [77].
FUTURE DIRECTIONS
To address the large role TCE (and other chlorinated solvents) may play in fueling the rise of PD, we need to do the following:
1. Conduct more research – Given the widespread environmental contamination by TCE, the authors of the twin study linking TCE to PD concluded, “the potential public health implications are substantial” [11]. Unfortunately, that prescient warning has largely gone unheeded. A search of TCE and PD on PubMed yields only 15 papers in the past decade [80]. By contrast, a search of the genetic risk factor GBA and PD returns more published papers in just the last two months [81]. Among the pressing research needs are evaluating cohorts (ideally prospectively) of individuals (likely) exposed to TCE, identifying biological markers of prior exposure, better understanding the mechanisms of injury, and assessing gene-environment interactions including those affecting TCE’s metabolism. Further work is also needed to estimate the risk of TCE exposure in conjunction with other known neurotoxicants, such as pesticides, and risk factors like traumatic brain injury.
2. Clean and contain contaminated sites – Hundreds of thousands of sites are contaminated across the U.S. and globally. They are found in strip malls where dry cleaners used to operate, on military bases where use was widespread, in cities near old manufacturing sites (especially those near rivers or streams), and in rural areas where landfills were created to dump hazardous waste. Fortunately, contaminated sites can be remediated, and homes, schools, and workplaces can be protected by vapor intrusion mitigation systems like those used for radon [82]. Until they are cleaned, existing contaminated sites must be contained, limiting exposure for humans and nature. Local, regional, and national authorities should take responsibility in overseeing rapid control of contaminated sites.
3. Monitor TCE levels and publicly communicate risk – Most databases monitor emissions, not current levels, and monitoring tends to be sporadic and reactive. TCE testing in groundwater, drinking water, soil, and in outdoor and indoor air should be widespread, frequent, and part of routine water quality testing. The results should be readily and publicly available. Polluted sites need to be marked as such, and the dangers to health clearly communicated to all parties at risk.
4. Ban trichloroethylene – In many ways, the long-established health risks of TCE dwarf its relationship with PD. TCE causes cancer, increases the risk of miscarriages, contributes to birth defects, and is associated with diseases in nearly every organ system. The chemical is over a century old. We do not fly airplanes from the days of the Wright brothers or drive cars from Henry Ford’s era; engineers have developed safer ones. Chemists can do the same for solvents. Some companies now advertise safer alternatives to TCE [83]. They are needed as the use of TCE continues to rise globally.
5. Listen to our patients – Finally, we should listen to our patients more. In medicine, we often move from diagnosis to treatment without considering the cause. The vast majority of individuals with PD do not have a family history of the disease or carry an identifiable genetic risk factor. Listening to their life stories or occupational histories can help identify TCE or other factors contributing to PD and could help develop etiology-specific treatments. This information can also inform their care (e.g., cancer screening), provide guidance to family members, co-workers, and classmates, and advance our understanding of the potential causes of this debilitating and likely very preventable disease.
CONCLUSION
For more than a century, TCE has threatened workers, polluted the air we breathe— outside and inside— and contaminated the water we drink. Global use is waxing, not waning. Most of this has been invisible, all of it is unacceptable, and none of it will stop until we act.
Recommend
About Joyk
Aggregate valuable and interesting links.
Joyk means Joy of geeK
![Trichloroethylene (TCE) chemical structure [84].](https://content.iospress.com/media/jpd/2023/13-2/jpd-13-2-jpd225047/jpd-13-jpd225047-g001a.jpg)
![Top ten exporters and importers of trichloroethylene, 2020 [33].](https://content.iospress.com/media/jpd/2023/13-2/jpd-13-2-jpd225047/jpd-13-jpd225047-g002a.jpg)
![Countries with published studies of sites of groundwater TCE contamination [89].](https://content.iospress.com/media/jpd/2023/13-2/jpd-13-2-jpd225047/jpd-13-jpd225047-g002b.jpg)
![U.S. cities that released the most TCE into the air, 1987 [35].](https://content.iospress.com/media/jpd/2023/13-2/jpd-13-2-jpd225047/jpd-13-jpd225047-g003a.jpg)
![U.S. cities that released the most TCE into the air, 2020 [35].](https://content.iospress.com/media/jpd/2023/13-2/jpd-13-2-jpd225047/jpd-13-jpd225047-g003b.jpg)