

部分肿瘤NGS检测相关公司2022-Q2财报比较
source link: https://kaopubear.top/blog/2022-08-08-2022q2-ngs-precision-oncology-revenue/
Go to the source link to view the article. You can view the picture content, updated content and better typesetting reading experience. If the link is broken, please click the button below to view the snapshot at that time.

暂时纳入了5家美国上市的公司,分别是Myriad, Natera, Guardant, Veracyte和Exact Sciences,Invitae将于8月9日发布,国内在美股上市的两家公司燃石医学和泛生子后续发布后将一并补充进来。
2022年8月8日这几家公司的股价如下图所示。
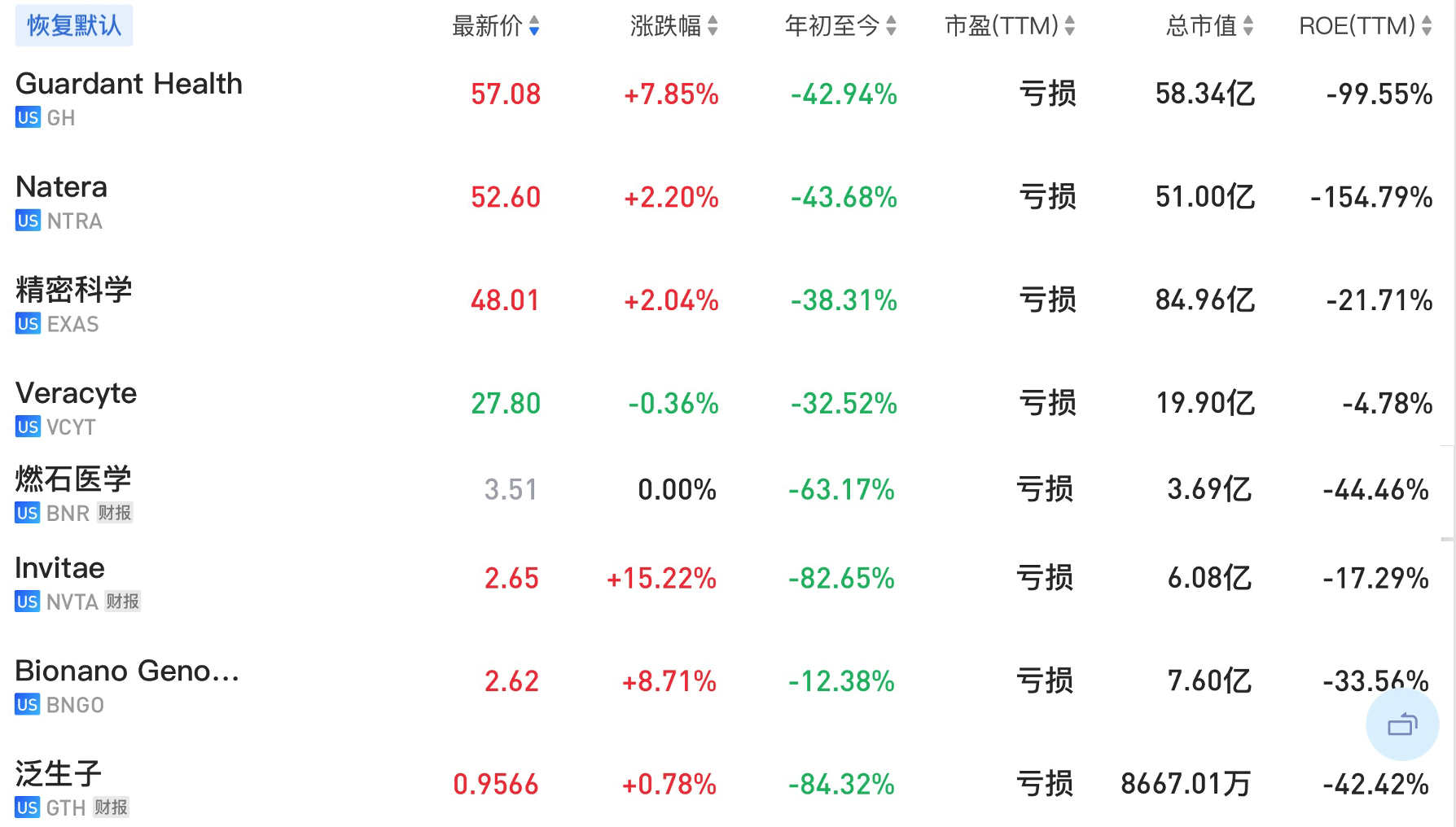
Myriad
News Release Detail | Myriad Genetics
Highlights:
- Revenue of $179.3 million for the quarter ended June 30, 2022
- Excluding revenue from divested businesses, revenue increased 7% year-over-year and 9% sequentially from the first quarter of 2022
- Diluted GAAP earnings per share (EPS) of $ (0.18) and adjusted EPS of $0.04 in the second quarter of 2022
- Fiscal year 2022 financial guidance updated to reflect additional $20 million investment in research and development, technology, and sales and marketing programs
- Ended the quarter with $283.6 million in cash, cash equivalents and investments
Oncology
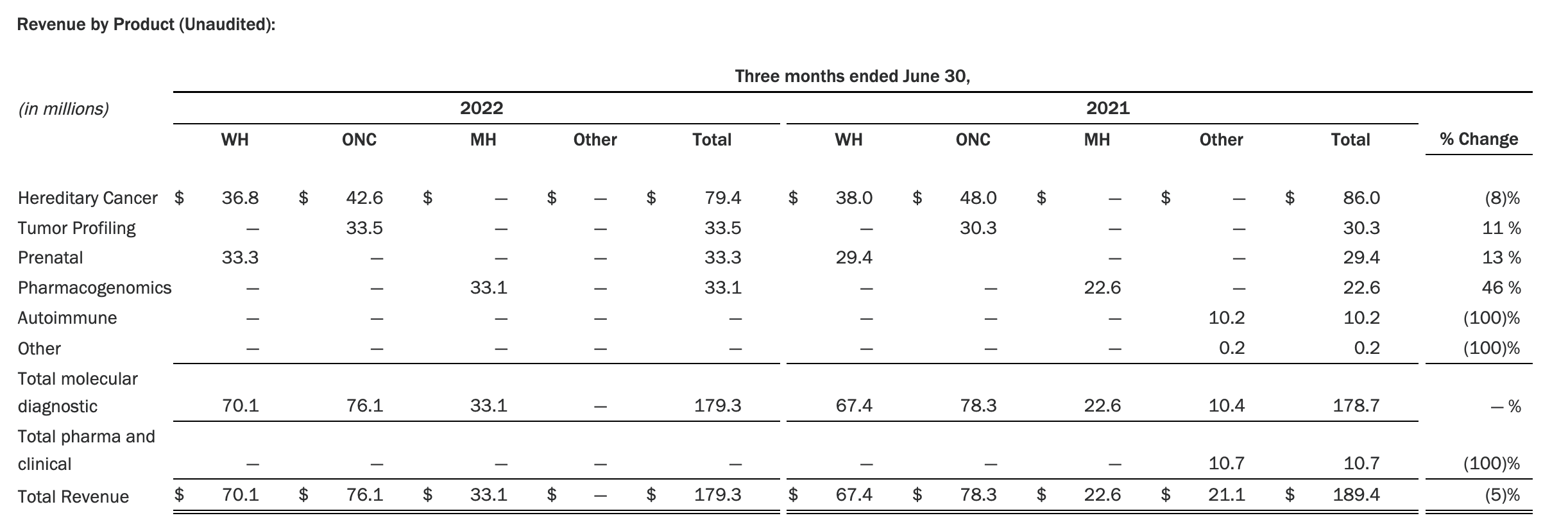
The Myriad Genetics Oncology business provides hereditary cancer testing, including the MyRisk™ hereditary cancer test for patients who have cancer. It also provides tumor profiling products such as the myChoice® CDx companion diagnostic test, the Prolaris® prostate cancer test, and the EndoPredict® breast cancer prognostic test. The Oncology business delivered revenue of $76.1 million in the second quarter of 2022, a decrease of 3% year-over-year and an increase of 9% sequentially from the first quarter of 2022.
- Myriad Genetics recently launched Precise Oncology Solutions, which combines the company’s MyRisk germline cancer testing technology and its myChoice CDx companion diagnostic test with Precise Tumor, a tumor profiling test powered by Illumina, Inc.'s TruSight™ Oncology 500 (TSO500) assay and processed by Intermountain Precision Genomics.
- MyChoice CDx reported its highest quarterly volume level in the United States ever in the second quarter of 2022 with quarterly volumes up 63% year-over-year and 10% sequentially from the first quarter of 2022.
- Prolaris is a prostate cancer prognostic test designed to assess prostate cancer aggressiveness. The Oncology business achieved its highest quarterly Prolaris volumes in the second quarter of 2022, beating its previous quarterly volume record by 6%.
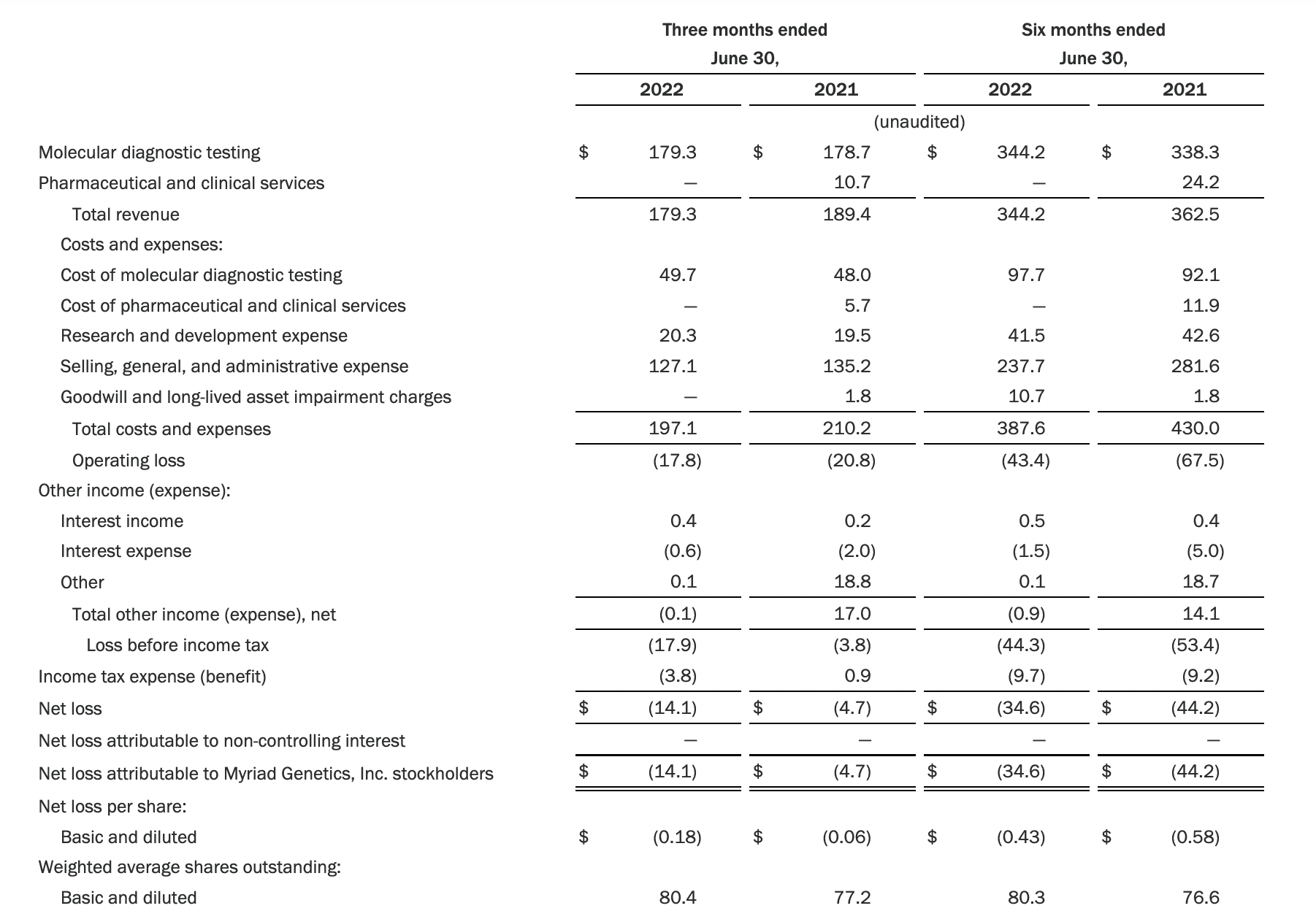
Myriad 2022年第二季度的收入同比下降了5%。该期间的总收入为1.793亿美元,低于上年第二季度的1.894亿美元。Myriad本季度净亏损1410万美元,而去年同期亏损470万美元。本季度末拥有1.052亿美元的现金和现金等价物。

Natera
Natera Reports Second Quarter 2022 Financial Results | Natera
- Generated total revenues of $ 198.2 million in the second quarter of 2022 compared to $142.0 million in the second quarter of 2021, an increase of 39.6%. Product revenues grew 39.3% over the same period.
- Processed approximately 499,900 tests in the second quarter of 2022, compared to approximately 375,700 tests processed in second quarter of 2021, an increase of 33.0%.
- 2022 revenue guidance raised to $ 805 million – $825 million.
- Selected to participate in UnitedHealthcare’s Preferred Laboratory Network after a rigorous review process.
- Publication of the Trifecta study for Prospera Kidney in Transplantation; largest prospective, multi-site, fully biopsy matched study to date.
- Completed enrollment in RenaCARE study for Renasight, with more than 1,700 patients across 30+ sites.
- Secured Medicare coverage for muscle invasive bladder cancer; fourth coverage decision for Signatera.
- Presented substantial new Signatera data sets at the 2022 ASCO Annual Meeting.
- Appointed Dr. Minetta Liu as CMO for Oncology.
- Additional equity investment in Natera by Executive Chairman Matt Rabinowitz.
第二季度总收入增长了40%,主要是受Signatera强劲增长的推动,特别是在结直肠和免疫疗法方面。收入增加到1.982亿美元,而去年同期为1.42亿美元。
2022年第二季度的产品收入增长39%,达到1.946亿美元,而去年同期为1.396亿美元。今年目前的进展使该公司有信心在2024年中期实现现金流平衡。
Natera公司第二季度的净亏损为1.452亿美元,而2021年同期的净亏损为1.16亿美元。
截至2022年6月30日,Natera持有约6.387亿美元的现金、现金等价物、短期投资和限制性现金(其中现金和现金等价物0.91亿),而截至2021年12月31日的现金为9.145亿美元(其中现金和现金等价物0.84亿)。
Guardant
Guardant Health, Inc. - Guardant Health Reports Second Quarter 2022 Financial Results
Recent Highlights
- Revenue of $109.1 million for the second quarter of 2022, an increase of 19% over the corresponding period of 2021
- Reported 29,300 tests to clinical customers and 6,000 tests to biopharmaceutical customers in the second quarter of 2022, representing an increase of 40% and 65%, respectively, over the second quarter of 2021
- Received Medicare Coverage for Guardant Reveal™, the first blood-only liquid biopsy test for molecular residual disease testing now covered for certain fee-for-service Medicare patients in the US with stage II or III colorectal cancer
- Completed the purchase of the Guardant Health AMEA Joint Venture, creating a unified organization to expand commercialization of Guardant Health's industry-leading liquid biopsy technology across the region
- Executed a strategic partnership agreement to offer comprehensive genomic profiling tests to biopharmaceutical companies in China with Adicon, a leading independent clinical laboratory company
- The Company’s Shield LDT launch was well received by clinicians and patients and with early activity exceeding expectations
- Expect both ECLIPSE readout and PMA submission for Shield assay during the second half of the year
截至2022年6月30日的三个月,收入为1.091亿美元,比截至2021年6月30日的三个月的9210万美元增长19%。精准肿瘤学收入增长了27%,主要是受临床检测量和生物制药样品量增加的推动,这两个数字分别比上年同期增长了40%和65%。
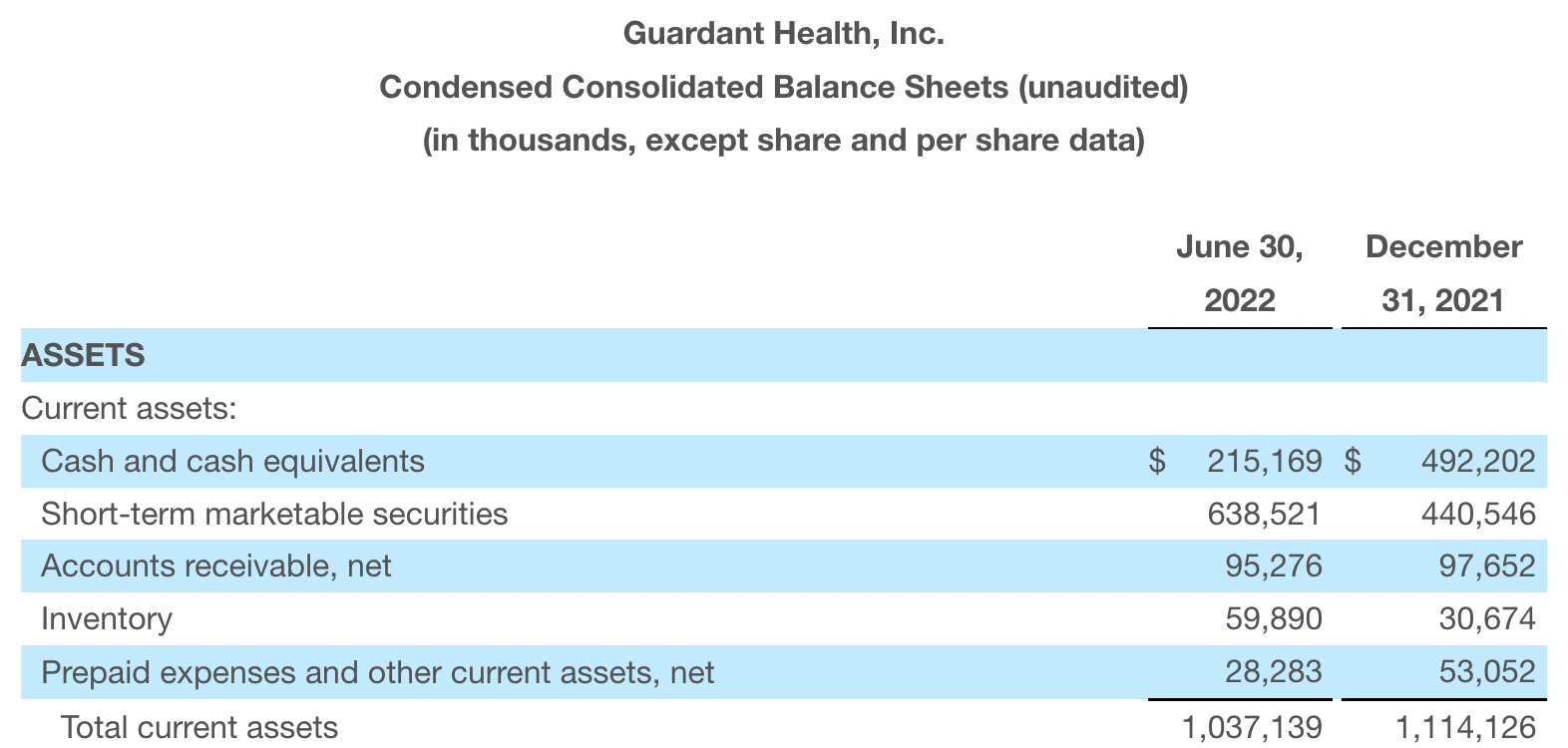
截至2022年6月30日,现金、现金等价物和有价证券为12亿美元。其中现金和现金等价物2.15亿美元,2021年底为4.92亿美元。2022 Q2 净亏损2.29亿美元,2021年同期为0.97亿美元。
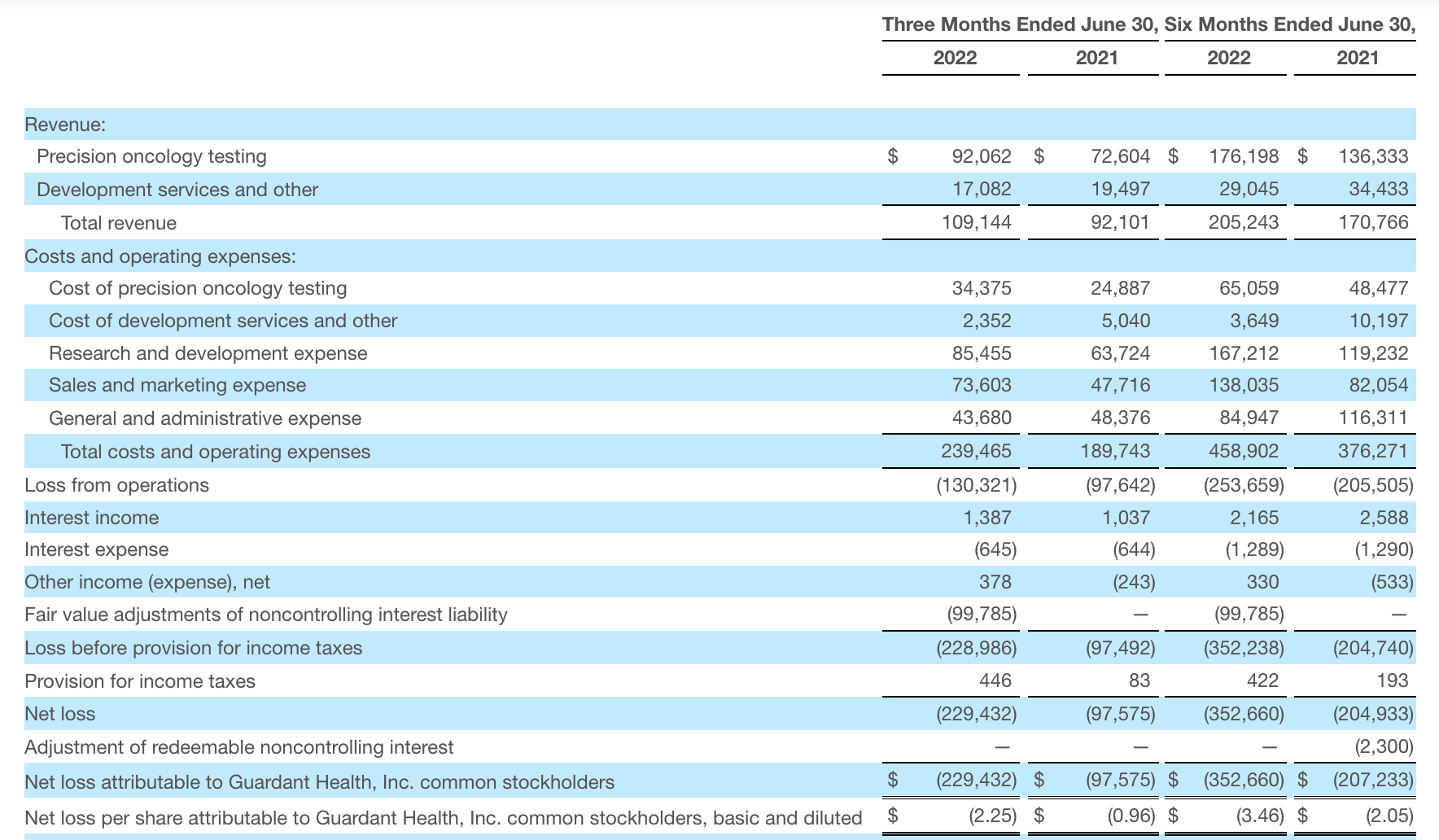
veracyte
Veracyte Announces Second Quarter 2022 Financial Results | Veracyte, Inc.
- Increased second quarter total revenue by 32% to $72.9 million, compared to the second quarter of 2021;
- Grew total test volume to 24,904, an increase of 19% compared to the second quarter of 2021;
- Announced that an updated clinical guideline from the American Urological Association and American Society for Radiation Oncology features a favorable statement for genomic testing, including Decipher Prostate, to help guide care for men with localized prostate cancer.
- Unveiled key clinical evidence across Veracyte’s portfolio:
- Decipher Prostate – Data was published in Annals of Oncology reinforcing the clinical utility of the Decipher Prostate genomic classifier for helping to guide the timing and intensity of therapy in men experiencing prostate cancer recurrence following radical prostatectomy. Additionally, data unveiled at the 2022 ASCO Annual Meeting demonstrated that population-based prostate cancer treatment patterns are independently associated with Decipher classifier score;
- Afirma Genomic Sequencing Classifier – Meta-analysis of independent, real-world studies were presented at ENDO 2022 demonstrating consistent and enhanced Afirma GSC performance, compared to the test’s original clinical validation study;
- Prosigna Breast Cancer Assay – New consensus survey data presented at the ESMO Breast annual meeting showed that leading breast cancer oncologists in Europe agree on the value of gene expression profiling tests, such as Prosigna, and on the importance of molecular subtype information to help inform treatment decisions for patients with early-stage breast cancer;
- Biopharma – New study findings presented orally at ASCO and in a paper published in Lancet Oncology showed the Immunoscore Immune Checkpoint (IC) biomarker’s ability to identify which patients will benefit from immune checkpoint inhibitors in metastatic non-small cell lung cancer and metastatic colorectal cancer, respectively; and
- Percepta Nasal Swab – Data presented at the ATS annual meeting showed that the noninvasive genomic test performed similarly well across the spectrum of tobacco-related risk.
- Ended the second quarter of 2022 with cash, cash equivalents and short-term investments of $164.0 million, compared to $166.4 million at the end of the first quarter of 2022.
2022年第二季度的总收入为7290万美元,与2021年第二季度的5510万美元相比增长了32%。检测收入为5970万美元,与2021年第二季度的5080万美元相比增长了18%,主要是由泌尿科检测的强劲表现所驱动。产品收入为310万美元,与2021年第二季度的270万美元相比,增长了16%。生物制药和其他收入为1,000万美元,与2021年第二季度的160万美元相比增加了840万美元,主要是由收购HalioDx的贡献所推动。
Veracyte第二季度的净亏损为950万美元,而去年同季度为900万美元。本季度末拥有现金、现金等价物和短期投资1.640亿美元。预计2022年全年总收入将达到2.72亿美元至2.8亿美元,同比增长24%至28%。
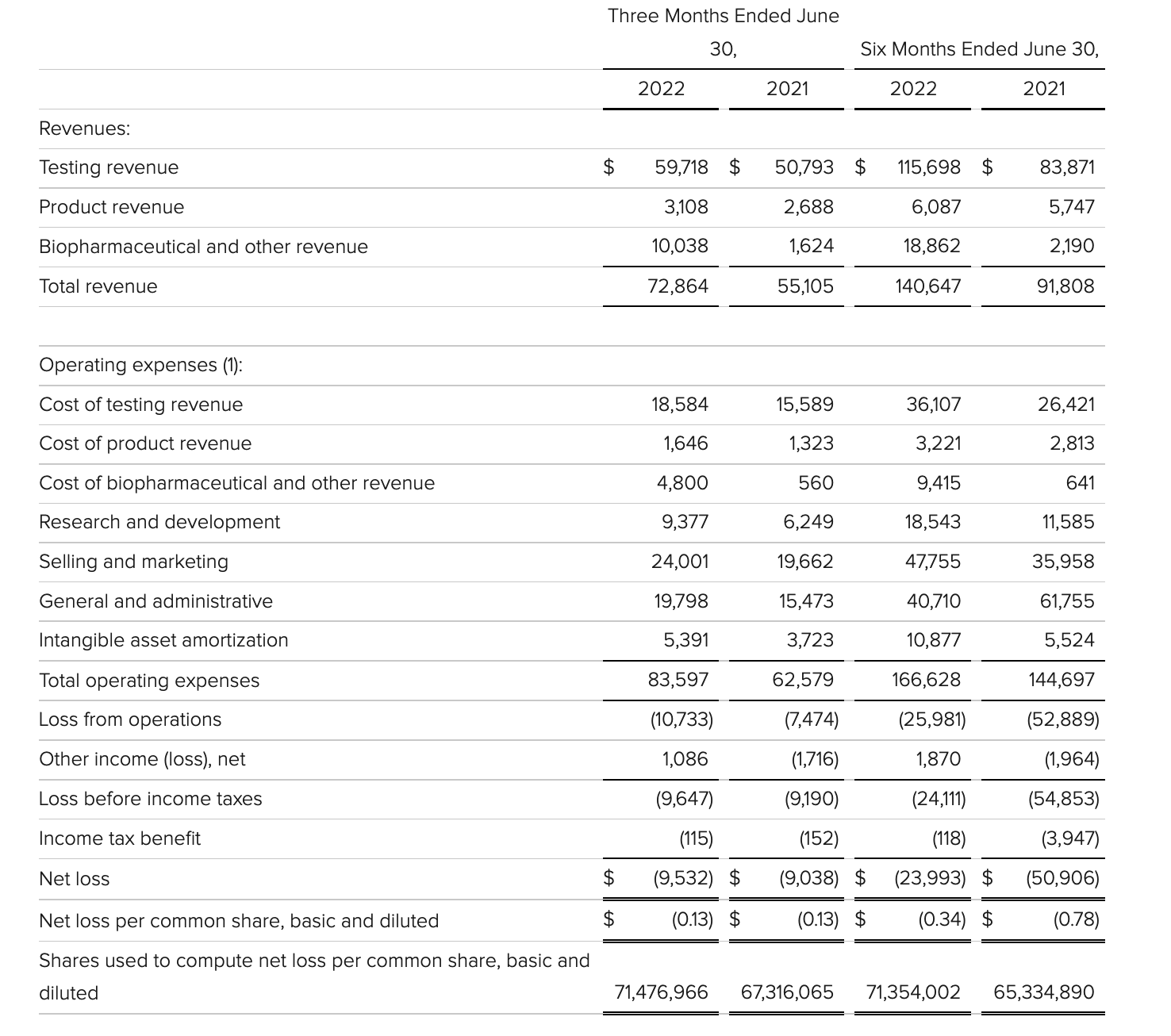
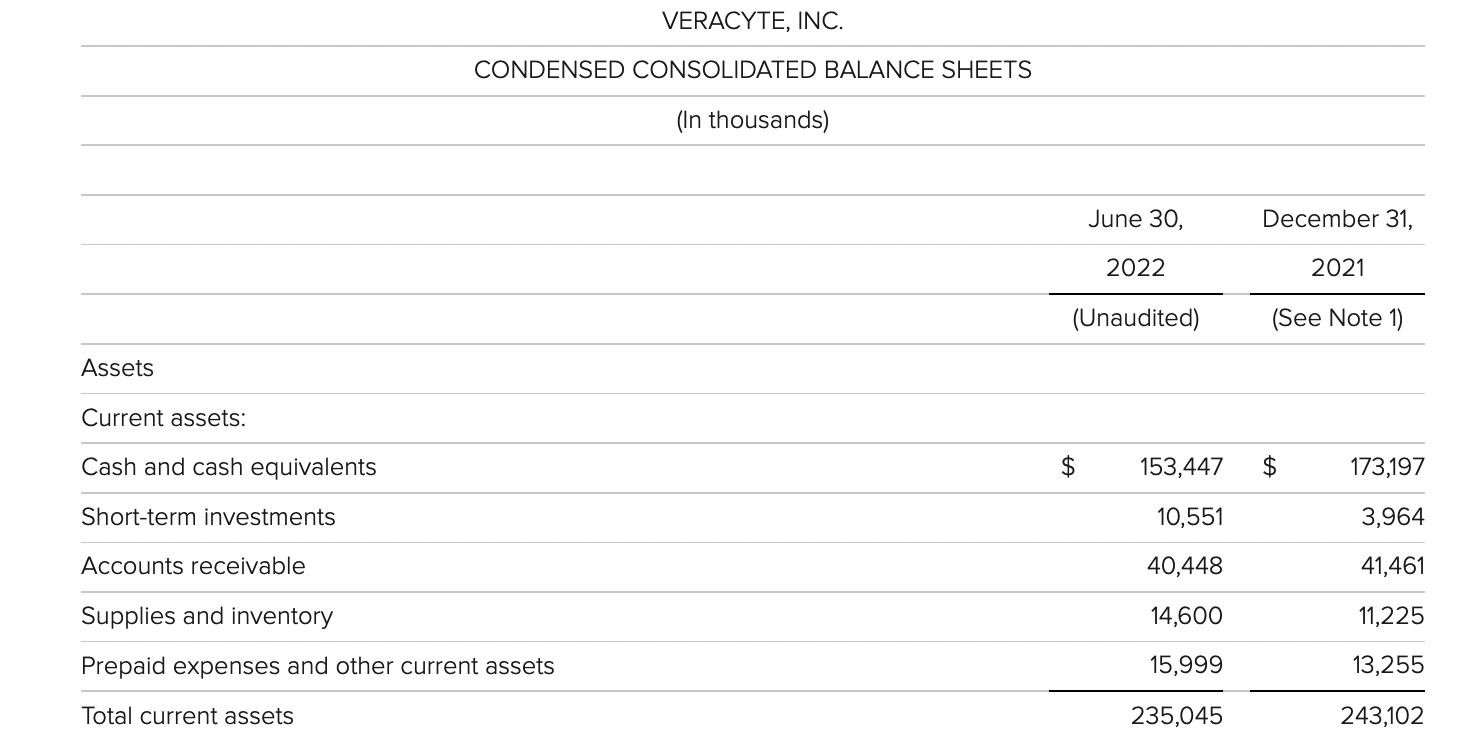
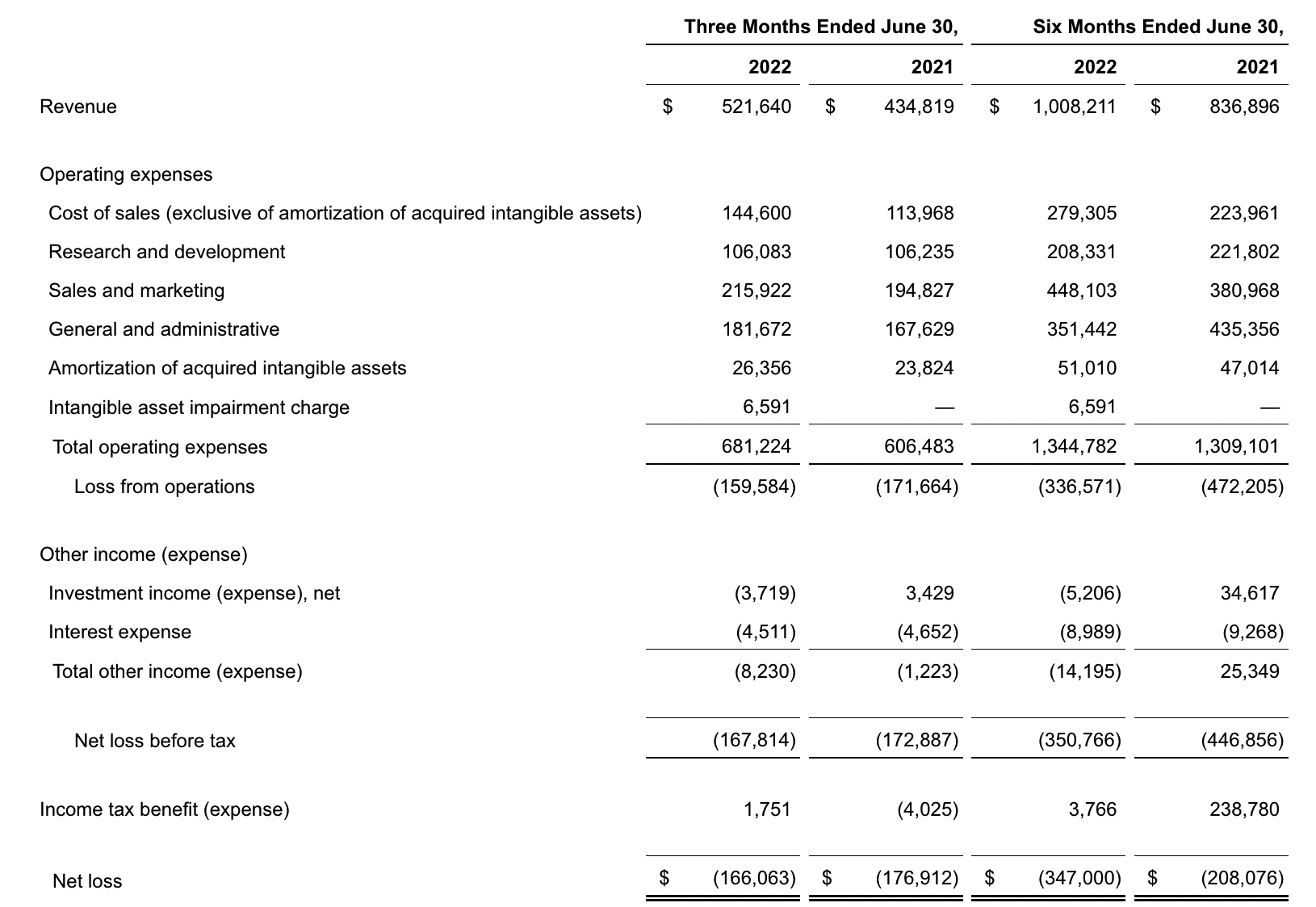
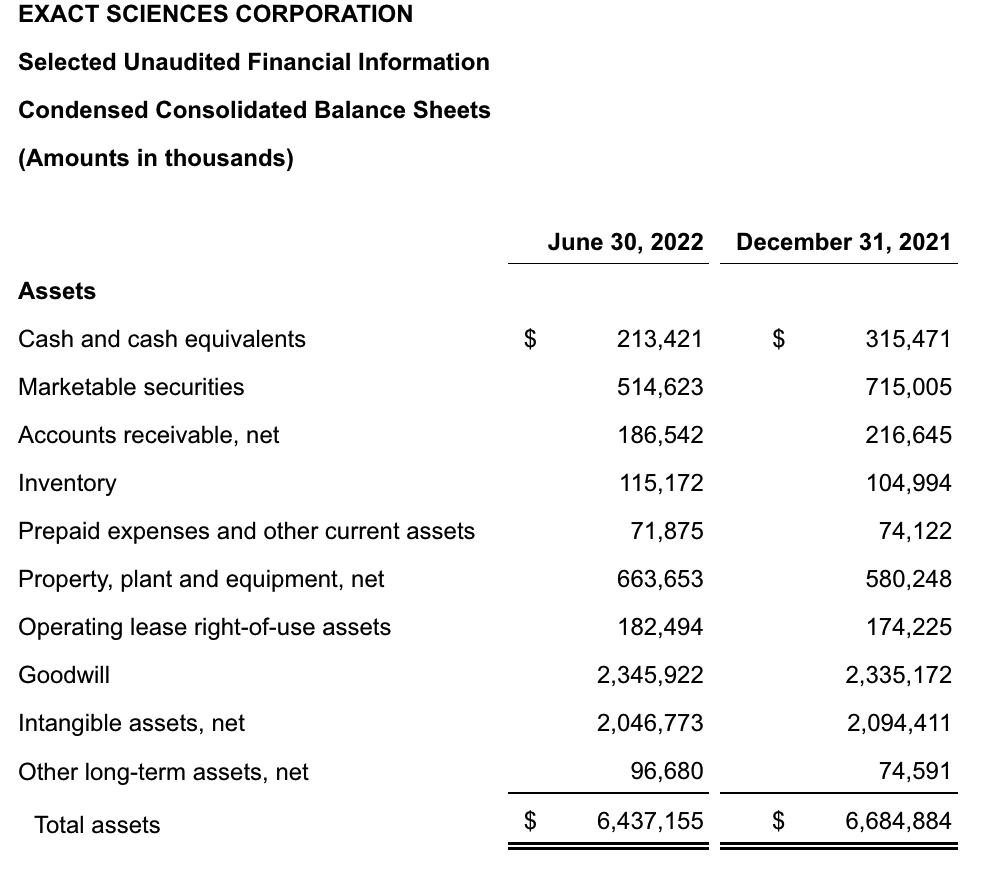
Exact Sciences
Exact Sciences Corporation - Exact Sciences Announces Second Quarter 2022 Results
- Total second quarter revenue of $522 million, including Screening revenue of $354 million, Precision Oncology revenue of $154 million, and COVID-19 testing revenue of $14 million
- Total second quarter revenue, excluding COVID-19 testing, increased 26 percent compared to the second quarter of 2021, including 34 percent increase in Screening revenue and 12 percent increase in Precision Oncology revenue
- Screening revenue growth driven by improved sales team productivity, Cologuard marketing partnership with Katie Couric, Cologuard rescreens, and Cologuard use in 45-49 age group
该公司第二季度的总收入为5.216亿美元,比2021年同期的4.348亿美元增长20%。肿瘤相关收入为1.54亿美元,同比增长约12%。
该公司宣布将放弃Oncotype DX在前列腺中的检测,以高达1亿美元的价格出售给MDx Health。
Exact公司第二季度的净亏损为1.661亿美元,而去年同期的净亏损为1.769亿美元。该公司第二季度末的现金和现金等价物为2.134亿美元,有价证券为5.146亿美元。
Invitae
August 9, 2022 发布
August 31, 2022 发布
未公布时间
本文作者:思考问题的熊
版权声明:本博客所有文章除特别声明外,均采用 知识共享署名-非商业性使用-禁止演绎 4.0 国际许可协议 (CC BY-NC-ND 4.0) 进行许可。
· 分享链接 https://kaopubear.top/blog/2022-08-08-2022q2-ngs-precision-oncology-revenue/
Recommend
About Joyk
Aggregate valuable and interesting links.
Joyk means Joy of geeK