

What Is a Supercritical Fluid?
source link: https://www.scimed.co.uk/education/what-is-a-supercritical-fluid/
Go to the source link to view the article. You can view the picture content, updated content and better typesetting reading experience. If the link is broken, please click the button below to view the snapshot at that time.

What is a Supercritical Fluid?
A supercritical fluid is a highly-compressed fluid that combines the properties of gases and liquids. Supercritical fluids have a range of supporting technologies used in several industrial applications.
They form the basis of clean technology as an alternative solvents for extracting natural products, chemicals and other substances.
How do you create a Supercritical Fluid?
Applying temperature and pressure above the critical point of a substance, pushes that substances into the supercritical phase. The properties of this fluid can be adjusted by going further into the supercritical region. This is achieved by increasing the temperature or pressure.
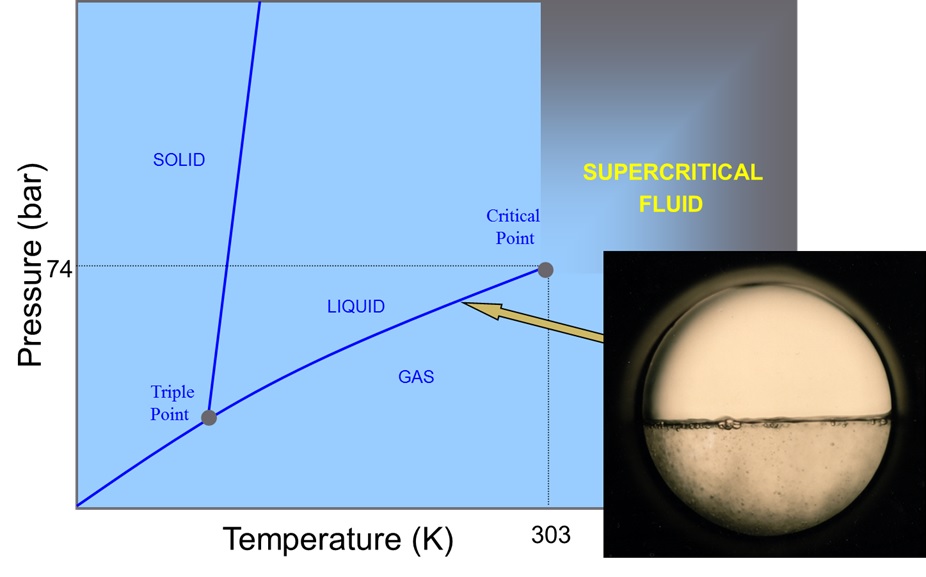
For example, increasing the temperature above 31°C and pressure above 73 bar for Carbon dioxide creates a supercritical phase, neither liquid nor gas but a combination of both properties. High diffusion like a gas but with the solvation (ability to dissolve substances) of a liquid. Further manipulation of these conditions can be used to create a variety of unique properties used to extract a number of natural products.
What are the properties of supercritical fluids?
Supercritical fluids, or SCFs, create fluids with:
- densities equivalent to that of liquids
- diffusion and viscosities exhibited by gases.
These properties create supercritical fluids that can be manipulated so be used in industrial processes by
- diffusing through solids like a gas, while
- dissolving materials like a liquid
With Carbon dioxide and water being the most commonly used supercritical fluid used within industrial applications. Other supercritical fluids can be created from the following substances:
- Carbon dioxide
- Water
- R134a
- Ethane
- Propane
- Ethylene
- Propylene
What is supercritical CO2?
Supercritical CO2 has the most accessible critical temperature and pressure at 31°C, 74 bar. This coupled with the following properties makes it one of the most versatile green solvent available:
- Non-toxic,
- Non-flammable
- Chemically inert (non-reactive).
- Low-cost while being available at high levels of purity.
This means you can use supercritical CO2 fluid at mild temperature conditions, between 40° and 60°C, and it will not leave behind harmful organic residues.

Subcritical and Supercritical water (H2O)?
Water has a higher supercritical point than CO2, at 374°C and 220 bar but it is well-suited to various hydrothermal processes, for instance oxidation in waste processing (wet air oxidation, or WAO) and processes to treat hazardous waste and synthesise nanoparticles.
At supercritical levels, oxidation reactions occur, and organic compounds and gases will mix together homogenously.
However subcritical water is commonly used as an equivalent or alternative to supercritical CO2. The subcritical region of water is highly compressed water that is still in its liquid state. By varying the temperature and pressure between 150°C and 350°C, and 15 to 200 bar the properties of the fluid can be alternated to selectively extract natural materials.
What are the uses of supercritical fluids?
Supercritical fluids can perform various processes, including:
- Extraction
- Separation
- Concentration
- Purification
- Sterilisation
- Impregnation
- Enrichment
- Chemical reactions
- Drying
For most solutes, supercritical Carbon dioxide solvent power is similar to that of light hydrocarbons, such as hexanes and pentanes. In fact, fluorinated compounds are more soluble in supercritical CO2 than in hydrocarbons. This solubility is an essential part of the polymerisation process.
In addition to extraction, SCF can be used to micronise materials. By dissolving a substrate in the supercritical phase then rapidly expanding the contents through depressurisation the dissolved materials rapidly precipitate. This rapid precipitation prevents material agglomeration or crystal growth. creating finely divided solids in the nano range.
Supercritical fluids also mix readily with gases, such as nitrogen or hydrogen. This leads to much higher concentrations of dissolved gases, compared to the effects achieved using solvents. This can be useful for synthetic alternations such as hydrogenations.
How do supercritical fluid processes work?
Here are more details about some of the major processes involving supercritical fluids.
Extraction
The main advantage of supercritical carbon dioxide (CO2) has over solvents used in extraction processes its high diffusion and the ability to selectively adjust the properties to selectively extract different components. Once dissolved in supercritical CO2 depressurisation releases the extracted components and the CO2 is returned to a gas This can then either be evaporated harmlessly into the air, or recycled by condensation.
Sterilisation
Supercritical fluids can be used to deactivate or destroy some bacteria, fungi, and yeasts through the high pressure disrupting or destroying the cell walls They are therefore used in sterilising food and medical instruments.
Impregnation
Supercritical fluid impregnation involves delivering the active substance onto an inert substrate, In its gaseous state, the CO2 leaves the target substance impregnated with the active substance. This enables the impregnation of solid substances with active compounds. Examples include textile dyeing, impregnating wood and tanning leather.
Purification
Chromatography of extracted substances using supercritical fluids can achieve high levels of purification Examples include vegetable oils, plant extracts, polymers and fish oil.
Chemical reactions
Supercritical fluids can enhance the solubility of reactants and products increasing reaction rates. Examples include biomass conversion, fuel processing, various forms of catalysis and polymerisation.
Drying
Due to an absence of surface tension in supercritical fluids, you can use these fluids to dissolve solvents into the supercritical phase without collapsing the 3D structures within highly porous materials. This leaves a dried solvent free product with an unaltered porous structure.
This process is also used in modern dry-cleaning techniques for clothes, to replace chlorinated solvents.
What are the industrial applications of supercritical fluids?
The technologies utilising supercritical fluids are involved in a great many industrial applications, across a broad spectrum of sectors.
Here are examples of industries where supercritical fluids provide essential functions:
- Food and Drink
The use of supercritical fluids as extraction solvents for organic food products has been long established.
Applications in the food and drink industry include coffee decaffeination, production of natural colourants, milk sterilisation and deodorising fish oils.
It is also used to ensure corks are free from cork taint, caused by the presence of the chemical trichloroanisole.
- Pharmaceutical
Supercritical fluids help in the processing of various plant extracts used in the pharmaceutical industry, such as diterpenes (antioxidants) and triterpenes (phytosterols). They are also used in the micronisation and impregnation of active pharmaceutical ingredients.
- Cosmetics
The cosmetic industry uses natural antioxidants in many of its products. Supercritical CO2 enables manufacturers to extract these antioxidants from a wide variety of fruit and vegetables. These antioxidants include: tocopherols (vitamin E), polyphenols and carotenoids. Supercritical fluids also extract floral fragrances from flowers.
- Plastics and Polymers
Supercritical CO2 is a good plasticising agent as it reduces the temperature during glass transition, improving the properties of resulting polymers. Diffusing supercritical CO2 in polymers changes their physio-chemical properties. Supercritical CO2 is also used to purify polymers from residual solvents, and to synthesise them with composites.
- Textiles
You can dye textiles using supercritical CO2 as an alternative solvent, and the same principles apply to tanning leather. In both applications, supercritical fluids provide solutions that are both economical and environment-friendly. Similarly, the use of supercritical CO2 in dry cleaning makes for a green alternative to chlorinated solvents.
What are the benefits of supercritical fluids?
As an alternative to organic solvents, supercritical fluids can contribute to a greener chemical industry and support environment-friendly industrial processes.
They provide the means for designing sustainable chemical engineering processes. Supercritical CO2 can remove polluting materials from the environment, such as pollutants in soil.
Other supercritical fluids can make environmental contributions: supercritical methanol helps in the recycling of plastic bottles, for example.
Supercritical fluids also have economic benefits for instance extraction using organic solvents, downstream processing contributes to production costs. This involves having to evaporate the solvent, and recover the extract. With solvent traces remaining in the extract that require more processing. Because supercritical fluid extraction processes use highly compressed gases such as CO2 recovery is much easier, more efficient and safer.
Supercritical fluids are versatile, with several applications in addition to extraction, applying across a broad range of industries. These applications are becoming more widespread as people realise the potential and value of supercritical fluids.
Are you looking for a green solvent?
Supercritical fluids provide versatile, green alternatives in key industrial processes and applications.
If you want to find out more about this green alternative to organic solvents, then please call us on +44 (0) 161 442 9963, email [email protected] or complete our online enquiry form.
Recommend
About Joyk
Aggregate valuable and interesting links.
Joyk means Joy of geeK